Mechanisms of specificity and communication in bacterial immunity
Our research group is currently focused on the following topics:
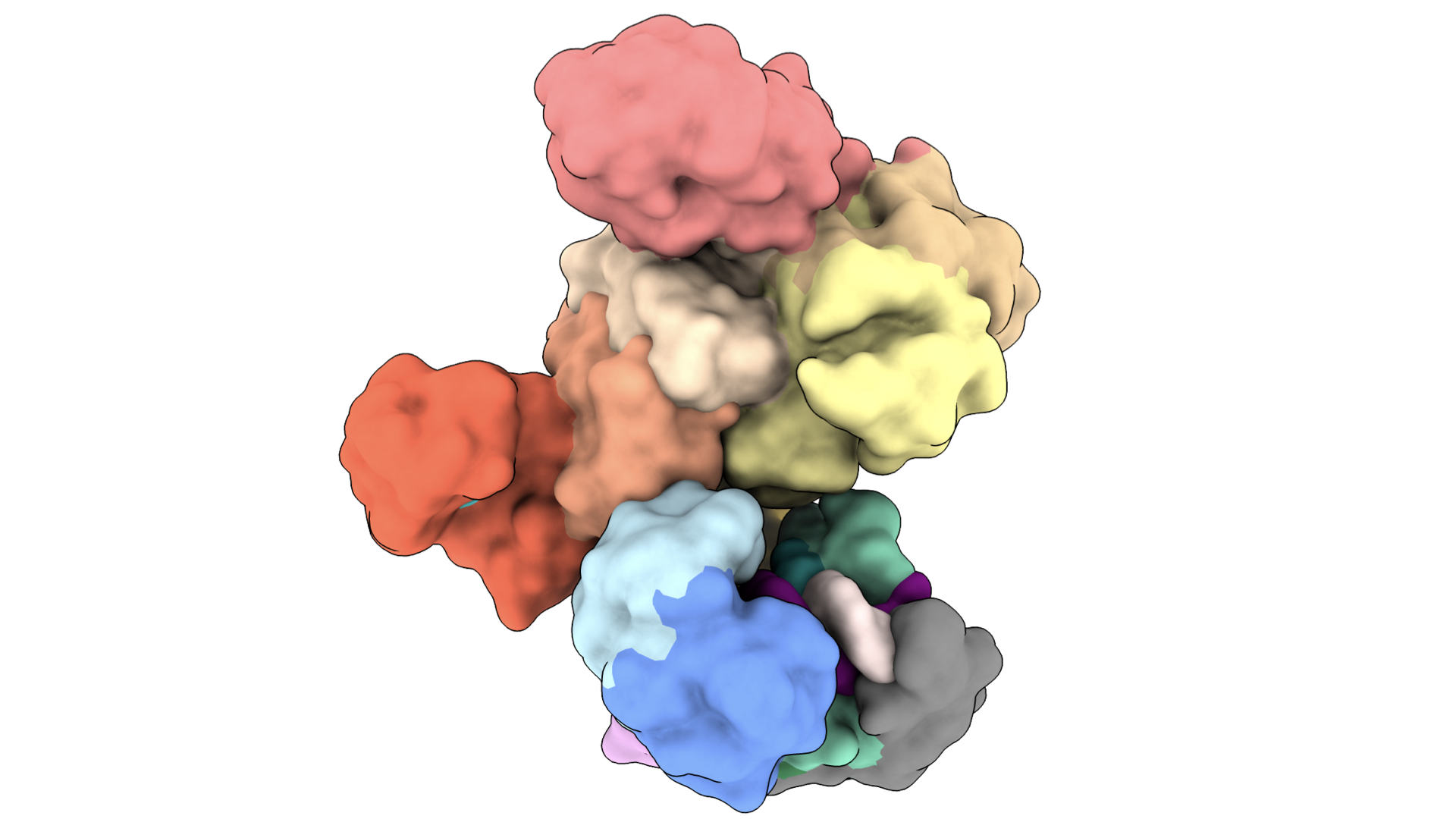
Structures of nucleoprotein complexes
We use a combination of cryo-EM, biochemistry and in vivo assays to understand how immune systems discern self-versus-non-self nucleic acids, and how these systems can be co-opted for novel biotechnology tools. See: Bravo et al., Nature, 2024, Bravo et al., Nature, 2023; Bravo et al., Nature Communications 2022
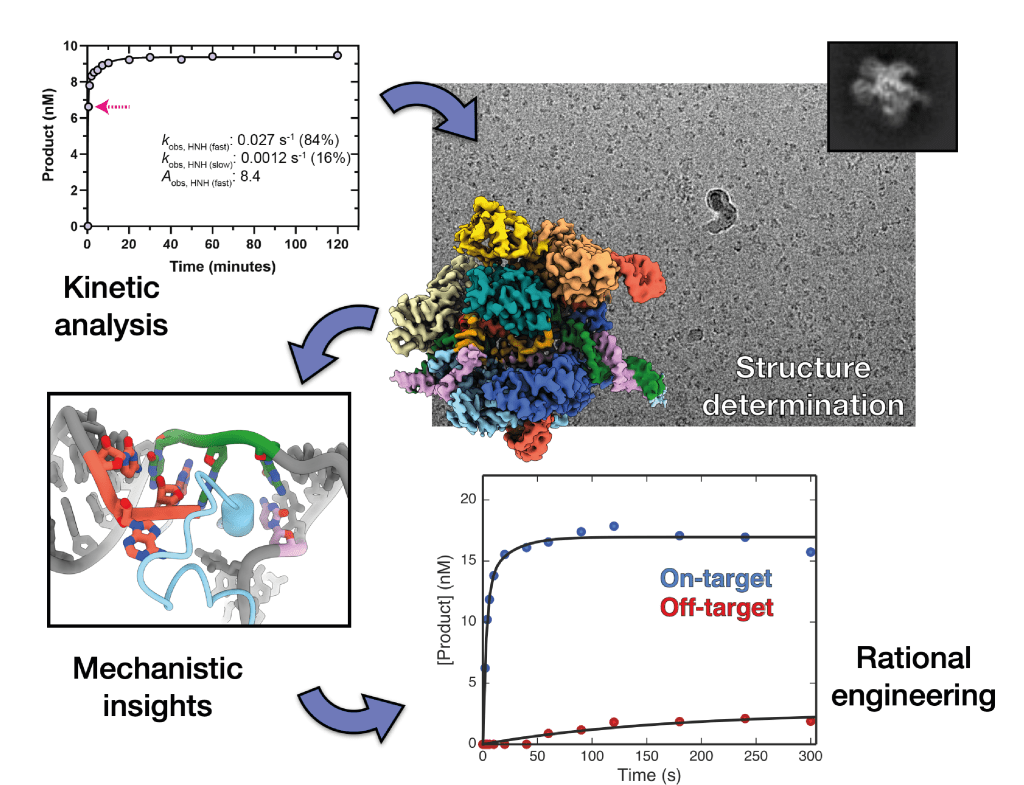
Cryo-EM methods development
Kinetics-guided structural studies to capture transient intermediate states of active complexes. See: Bravo et al., Molecular Cell, 2021; Bravo et al., Nature, 2022; Hibshman et al., Nature Communications, 2024
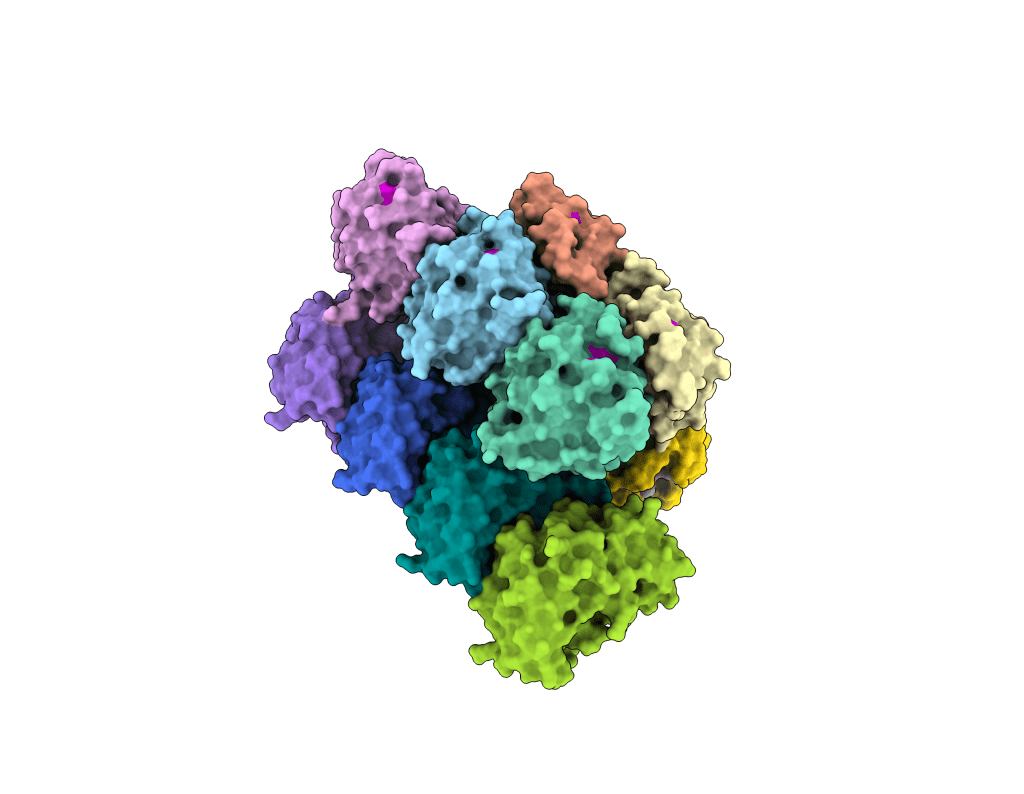
Bacterial Communication Networks
We use cryo-EM and kinetics to study how distinct bacterial immune systems communication through signalling molecules, how signal transduction determines cellular fate in response to infection, and how ancient Caspase enzymes have been adapted for metazoan inflammation and apoptosis. See: Steens, Bravo & Salazar et al., Science, 2024
Bacteria and parasitic mobile genetic elements (including phage and plasmids) are in an ongoing arms race. This tremendous evolutionary pressure has driven the development of a myriad of sophisticated bacterial defense systems capable of providing immunity. While well-known systems such as CRISPR-Cas have been extensively studied, the rise of metagenome analysis has unveiled a plethora of enigmatic defense mechanisms with unknown mechanisms. These systems must distinguish self from non-self molecules, requiring exquisite specificity to avoid unintended activation and autoimmunity.
The Bravo group is particularly interested in understanding how diverse bacterial immune systems can identify self from non-self nucleic acids, and the consequences of activation. We use a combination of structural biology (typically cryo-electron microscopy), biochemistry, biophysics and functional assays to understand the molecular mechanisms that underpin immunity. Our emphasis on cryo-EM and pre-steady state kinetic analysis enables the visualization of transient intermediate conformational states, providing insights into the dynamic and heterogeneous nature of active enzymes.
Our ultimate goal is not only understanding these systems but harnessing that knowledge for rational structure-based engineering. Through this approach, we aim to redesign these defense systems, tailoring them to possess specific, desirable properties. These engineered systems typically exhibit enhanced efficiency and specificity, transforming into potent tools for genome editing. By tapping into the diverse enzymatic activities of newly discovered defense systems, we aspire to unveil a new arsenal of precision tools for molecular biology, poised to shape the future of biotechnological advancements.